Knowledge of normal glucose metabolism and basic pathophysiology of diabetes can help educators:
-
Explain diabetes to clients
-
Understand the actions of diabetes medications
Topics
This section:
Related:
Carbohydrates in cereals, grains, fruit, starchy vegetables, milk, yogurt and sweets are converted into glucose with the help of digestive enzymes. The exception is fibre, which is not converted into glucose. Glucose is then absorbed into the blood stream.
Fat and protein intake usually have minimal immediate effect on blood glucose values. However, for those with type 1 diabetes, studies have shown that protein and fat can result in postprandial hyperglycemia. Meals high protein and fat may require additional insulin delivered over several hours.
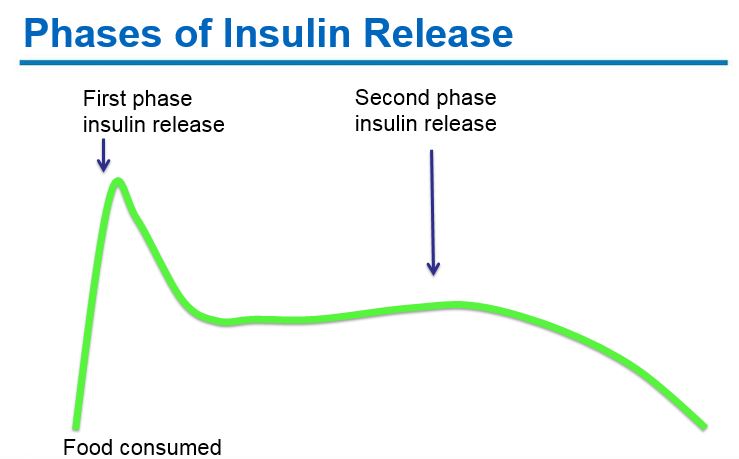
-
Early phase insulin release: Pancreatic beta-cells produce insulin in response to rising glucose levels. Proinsulin (the precursor molecule) is cleaved into c-peptide and insulin. A “first phase insulin response” occurs within 2 minutes of glucose arriving in the blood stream and continues for ~10-15 minutes. This “first phase insulin” prevents post-prandial hyperglycemia.
-
Second phase insulin release: A second phase of insulin release continues until blood glucose levels return to normal.
Incretin release: In response to glucose in the small intestine, gut hormones known as incretins stimulate insulin secretion, inhibit glucagon secretion, slow gastric emptying and induce satiety.
Glucose Uptake: Insulin binds to insulin receptors on cells, stimulating the uptake of glucose into cells - with the exception of brain cells, which do not require insulin to uptake glucose. Once inside the cells, glucose is used for energy or converted to glycogen (a storage form of glucose) or fat. Insulin also inhibits glucagon release and subsequent production of glucose by the liver.
Glucose uptake (sensitivity) is often improved with physical activity and weight loss.
-
Insulin’s main function is to lower blood glucose levels. Insulin is released in response to rising blood glucose from carbohydrate consumed and/or glucose produced/released from the body (mainly from the liver).
-
Insulin secretion is suppressed in response to dropping blood glucose levels.
-
Between meals, blood glucose levels are maintained through a balance between the production/release of glucose mainly from the liver (under the influence of counter-regulatory hormones) and the regular release of small amounts of insulin (basal insulin). The counter-regulatory hormones - glucagon, catecholamines (epinephrine and norepinephrine), glucocorticoids and growth hormone - stimulate the release of stored glucose (glycogenolysis) and the synthesis of glucose from non-carbohydrate precursors such as amino acids, glycerol and pyruvate (gluconeogenesis) mainly from the liver. This glucose release/production occurs between meals, in response to dropping glucose levels and stress (illness, injury, psychosocial stress etc).
-
Normally, the body prevents hypoglycemia by shutting off insulin release and by secreting the counter-regulatory hormones: glucagon, catecholamines (epinephrine and norepinephrine), glucocorticoids and growth hormone. Together this prevents low blood sugars by several mechanisms including encouraging the individual to ingest food, stimulating the release of stored glucose primarily from the liver (glycogenolysis) and synthesizing glucose from non-carbohydrate precursors such as amino acids, glycerol and pyruvate predominantly in the liver (gluconeogenesis).
-
The reabsorption of glucose. The kidneys reabsorb approximately 90% of filtered glucose in the early proximal tubule as mediated by sodium/glucose cotransporter 2 (SGLT-2).
Type 1 Diabetes
- Insulin deficiency due to beta cell destruction
- Exposure to unknown environmental factors which trigger the autoimmune response
- Beta-cells mistakenly recognized as "foreign" and attacked by patient's own immune system (T-cells)
- Genetically determined susceptibility factors
LADA: Latent Autoimmune Diabetes of Adulthood
- Progresses to insulin therapy more rapidly than type 2
- 2-12% of diagnosed diabetes
- Often misdiagnosed as type 2 diabetes
- Agents other than insulin often used at diagnosis
- Insulin eventually becomes necessary with beta cell failure
- There are no standard criteria for diagnosis of LADA.
Patient characteristics of LADA may include:
- Younger adult, but not always
- Lean body habitus, but not always
- A more rapid onset of hyperglycemic symptoms
- Presence or family history of autoimmune diseases
- Presence of anti-glutamic acid decarboxylase (anti- GAD) antibodies and anti-islet cell
Type 2 Diabetes
- Varying degrees of insulin resistance with relative insulin deficiency
- The body is unable to respond to insulin (insulin resistance)
- Reduced glucose uptake by muscle and fat cells
- Increased glucose release and production by the liver
- The body is unable to make enough insulin (beta cell dysfunction)
- Note:
- Genetic and environmental factors contribute to both insulin resistance and beta cell dysfunction.
- Obesity and lack of adequate physical activity can significantly increase insulin resistance.
- Prevention trials have shown reductions of up to 60% in the development of type 2 diabetes with weight loss and physical activity.
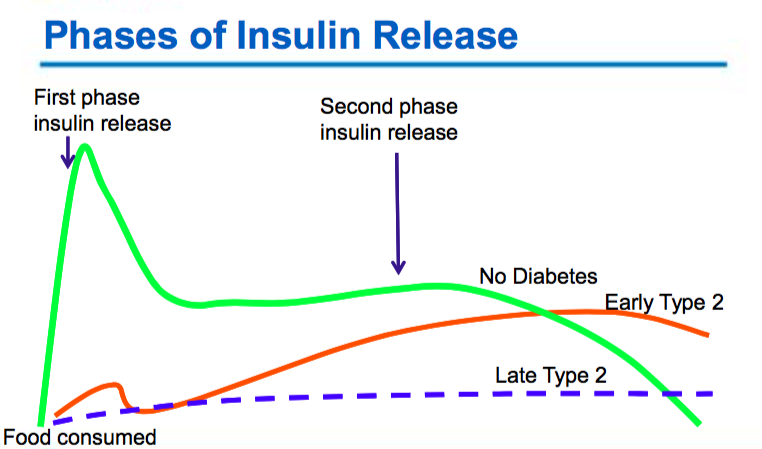
Gestational Diabetes (GDM)
- Glucose intolerance in pregnancy
- Increased insulin resistance secondary placental hormones
- Inadequate insulin secretion for increased insulin needs
- Potential progression to diabetes >30% over 15 years
Secondary Diabetes
- Diabetes as a result of another medical condition (pancreatitits, cancer, cystic fibrosis, medications, accident)
- Secondary causes vary resulting in varied treatment and/or duration of diabetes
- See Diabetes Canada, Etiologic Classification of Diabetes Mellitus
Symptoms of Diabetes Explained
| Sign/Symptom | Explanation |
|---|---|
| Glucosuria (glucose in urine) | Once the blood glucose level reaches approximately 10mmol/L, glucose is excreted in the urine. This "renal threshold" tends to be lower in children, pregnant women and the elderly. |
| Polyuria (excess urination) | Glucose in the urine has an osmotic effect pulling water with it, resulting in increased urine production. |
| Dehydration | Dehydration occurs if there is inadequate fluid replacement. |
| Polydypsia (increased fluid intake from excess thirst) | Secondary fluid loss. |
| Blurred vision | The lens and retina are exposed to hyperosmolar fluids which change the curvature of the lens. |
| Weight loss | Secondary to glycosuria and potentially dehydration. |
| Polyphagia (increased appetite) | The body attempts to make up for perceived lack of fuel. |
| Fatigue and weakness | Secondary reduced glucose entering cells and possibly lowered plasma volume. |
| Irritability, mood changes, headache | Brain cells are sensitive to blood glucose changes as they don't require insulin to uptake glucose. |
| Tingling and numbness to extremities | Effect of chronic hyperglycemia on the peripheral sensory nerves. |
| Delayed healing of wounds | Inhibited WBC function, poor tissue perfusion, and possible infection. |
| Muscle cramps in legs/feet | May be due to electrolyte disturbances, circulatory concerns, neuropathy. |
| Infections: Boils, abscesses, bladder, vaginal and penile yeast infections, surgical incision infections | Hyperglycemia and glycosuria both favor the growth of micro-organisms and yeast. |